naf lattice energy
As a result the lattice energy of NaF. Solvent data including KfKb Solubility data.
 |
| Example Lattice Energy Physical Properties Youtube |
Wiki User 2015-06-04 143317 This answer is.
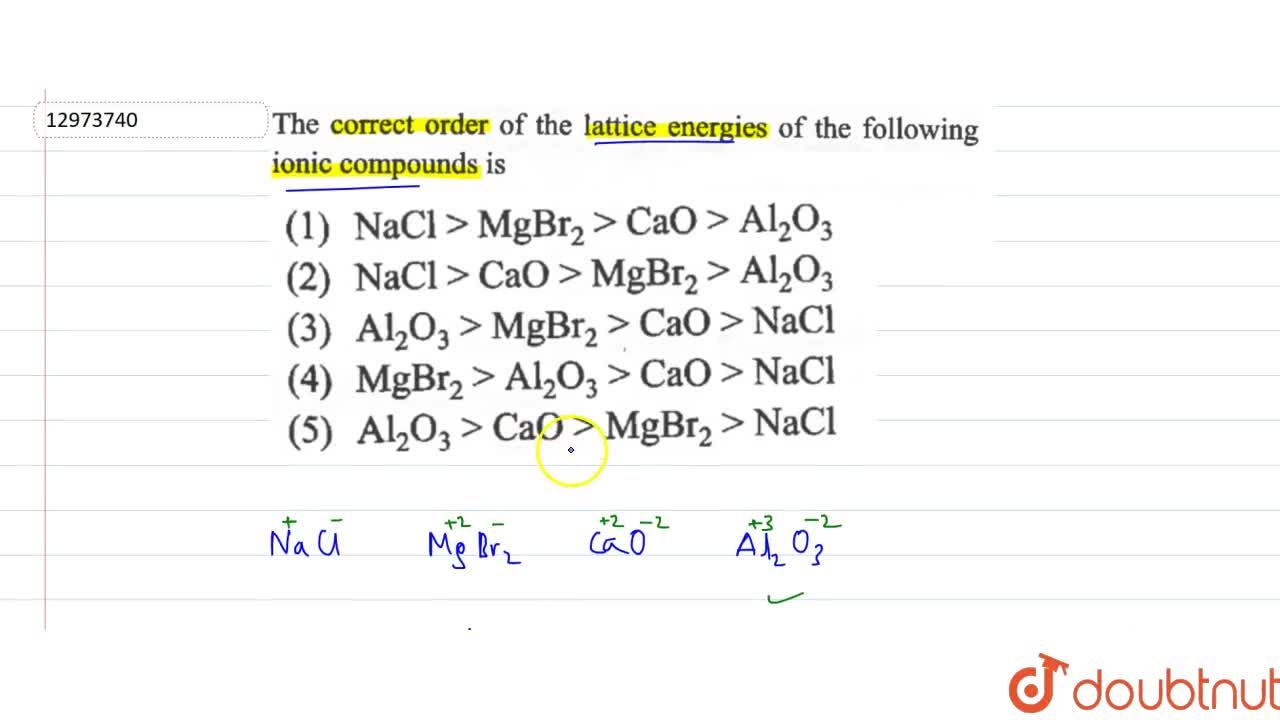
. The value of lattice energy of Na3N is -4422 kjmole and that of NaF is. Larger the magnitude of charge on ions greater will be the attractive forces higher is the value of lattice energy and inversely. Among the given compounds N aCl has higher r r. We would anticipate that the lattice energy of M gO formed from M g2 and O2.
816 rows Equivalently lattice energy can be defined as the amount of work energy that is released. For the reverse process of Equation 1. 2 a M b g b X a. The stability of an ionic.
Sodium Fluoride NaF has the highest lattice energy due to the smaller size of the cation. Lattice energy depends upon the charge density and ionic radii of the ions. As defined in Equation 1 the lattice energy is positive because energy is always required to separate the ions. Lattice energy is directly proportional to charge on ion ie.
Stronger the ionic bond formation higher the attice energy. Click hereto get an answer to your question Arrange the following as specified. So the correct order is RbCl KCl. LiF NaF MgO CaO LiF.
The lattice energy depends upon the relative sizes of the ions. 4 rows LiF has a lattice energy of 1030 kJmol NaF has a lattice energy of 910 kJmol and KF has a. Redox Coordination Kf. The lattice energy of NaF is -926 kJmol.
Since the is ionic charge of N3-ion greate r than F - hence Na3N compound has high lattice energy than NaF. Solution The correct option is C RbCl KCl NaCl NaF We know that lattice energy decreases as size of the ions in the compound increases. Study guides Chemistry 20 cards How does a buffer work What happens in a neutralization. The lattice energy of NaF is greater because of less relative size difference between the ions then LiF.
Lattice energy is energy released when a lattice forms. Lattice energy 1 rr. Lattice energy is the energy required to break 1 mole of an ionic crystal. Observe that anion is not very large.
We are asked to assess lattice energy with no other data but the formula of each salt. Therefore the distance between Na N a and F - F ions in NaF is less than the distance between the Cs C s and I - I ions in CsI. Which Ionic Bond has Highest Lattice Energy. Question 9 1 pts Arrange the ionic compounds in order of increasing lattice energy.
The smaller the size of ions the more will be the. LiF NaF KF RbF and CsF in order of increasing lattice energy Solve Study Textbooks Guides.
 |
| Ionic Bonding Lattice Energy Ppt Download |
 |
| Lattice Energies Ap Material Ppt Download |
 |
| Solved 1 C Predicting The Relative Lattice Energy Of Binary Ionic Compounds In Each Row Pick The Compound With The Bigger Lattice Energy Note Lattice Energy Is Always Greater Than Zero Which |
 |
| Solved The Lattice Energy Of Lif Is 1023 Kj Mol And The Li F Distance Is 200 8 Pm Naf Crystallizes In The Same Structure As Lif But With A Na F Distance Of 231 Pm |
 |
| Lattice Enthalpy Lattice Energy |
Posting Komentar untuk "naf lattice energy"